Respiratory superbugs
Pseudomonas aeruginosa is a type of bacteria that can infect people with long-term lung problems. If it isn’t quickly treated with the right antibiotics, the bacteria can form protective layers called biofilms. These biofilms make the infection very hard to get rid of.
Over 400 million at risk worldwide
People with COPD and bronchiectasis are especially vulnerable to respiratory superbugs.
Repeated hospital visits
Respiratory superbugs that are not treated with the right antibiotic can cause permanent lung damage, increasing healthcare costs.
PaST Kit: Rapid testing at the point of care
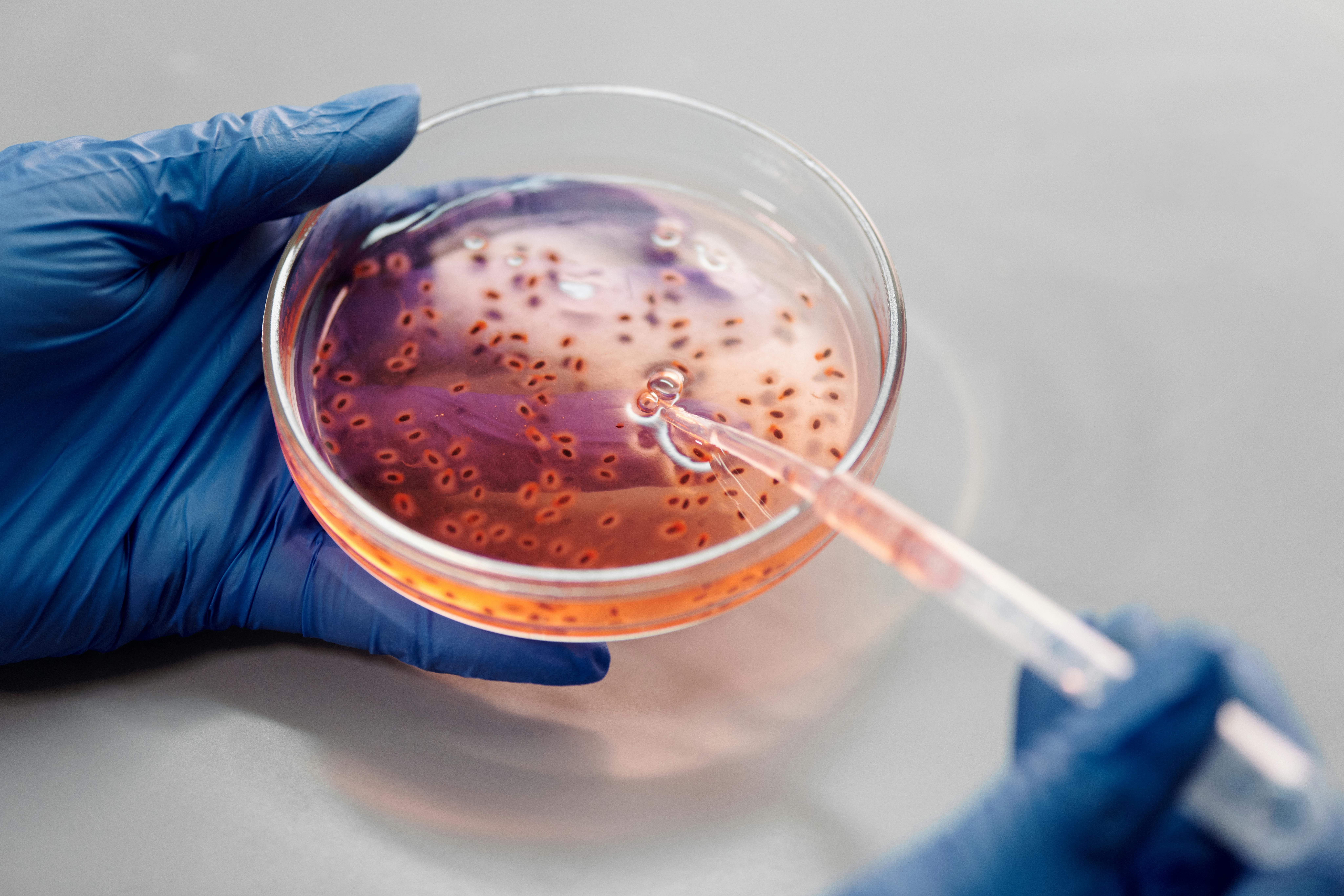
Nanodecal brings the first diagnostic kit that detects bacteria in sputum on the spot, right during your consultation. A reagent liquefies the sample, and a rapid test detects bacteria. These kits can be used anywhere in healthcare, making them ideal for guiding antibiotic treatments.
Faster than the standard of care
Culturing sputum takes several days. Nanodecal offers the first diagnostic kit to identify bacteria in sputum during the time required for a consultation.
Enabling precision
Nanodecal’s rapid test identifies bacteria quickly at the doctor's office, enabling the early administration of personalized treatments
Swiftlys® Sample liquefaction for decentralized analyses
Sputum, which contains bacteria that cause lung infections, is thick and nearly solid, making point-of-care analysis difficult. Nanodecal solves this problem with a reagent that liquefies sputum in just 2 minutes. Patent WO2021224529A1
A new antigen test for rapid lung diagnosis
Antibody-coated nanoparticles are contact-transferred, overcoming the limitations of lateral flow tests. They are produced using our proprietary method, protected under patent WO2021048087A1.
Designed for high sensitivity and specificity
Thanks to the simultaneous detection of multiple antigens of the same pathogen.
Our Team
We are putting together a multidisciplinary team to bring Nanodecal's tech from bench to market.

Roberto de la Rica
CEO | Founder
Steven M. Russell
Quality & Regulatory Affairs Manager
Stephanos Papaefstathiou
COO
Isabel Pérez Guillén
CSO
Xavier Riba
CFO
Cristina Adrover
R&D Director| Founder
Ana Losada
CTO
Francisco Moyano
Research AssistantAdvisory Board

Marcio Borges
President of the Code Sepsis Foundation and Director of the Sepsis Area at FEPIMCTI

Borja García-Cosío
Coordinator of the National Strategy on COPD for the Balearic Islands

Antonio Oliver
Head of Clinical Microbiology at HUSE and world expert in Pseudomonas infections

Antonio Ornelas
Advisor for Life Sciences & Medical Technology for Venture Capital Co.





.png)








